Overview
At-home tests reflect a significant shift from traditional approaches to diagnostic testing through laboratories. This shift in how and by whom diagnostic testing is performed needs to be accompanied by a shift in how test results are reported. Unlike laboratory test results that are managed by well-established laboratory information systems, technologies that manage results from at-home tests are still nascent.
RADx® MARS provides a solution for implementing standardized reporting of at-home tests based on two principles: (1) encoding of results and associated data in a healthcare industry-standard format and (2) identifying one (or a small few) destination(s) where these results can be sent and subsequently re-transmitted to appropriate state and federal health systems.
On this page:
- Learn more about RADx® MARS
- Data Flow
- Digital Testing Applications
- Data Hubs
- Data Communications Standards
- Mobile Application Guidance
- MakeMyTestCount
We invite you to share comments about the site, the HL7v2 Implementation Guide, or other feedback.
Learn about RADx® MARS
RADx MARS leverages industry-wide HL7v2 communications standards and is helping to lead a future transition to more versatile Fast Healthcare Interoperability Resources (FHIR).
HL7v2 resources include a detailed implementation guide, tools for identifying test-specific field parameters, an application programming interface, and a message validator developed by NIST.
The HL7v2 IG provides detailed instructions and requirements for every message component, ensuring compliance with state and federal requirements.
FHIR support includes an implementation guide using base resources.
A validation tool enables developers to test sample MARS HL7v2 messages against the Implementation Guide.
RADx MARS offers an application programming interface (API) that developers can use to construct, validate, and transmit HL7v2 messages.
The MARS HL7v2 specification is supported by two data hubs. These hubs simplify the process of routing test results to the appropriate state and federal health systems.
RADx MARS provides guidance for building mobile applications that capture test result reporting data from users.
RADx MARS provides an approach for de-identifying HL7v2 messages before they are transmitted to federal health systems.
Home test results can be sent to multiple destinations, which may then re-route to secondary systems. The standards approach ensures that results can be de-duplicated.
Current FDA EUAs for home tests stipulate the need to built result reporting solutions. RADx MARS standards and workflows conform to these requirements.
Frequently asked questions have been posted about the RADx MARS program.
Data Flow
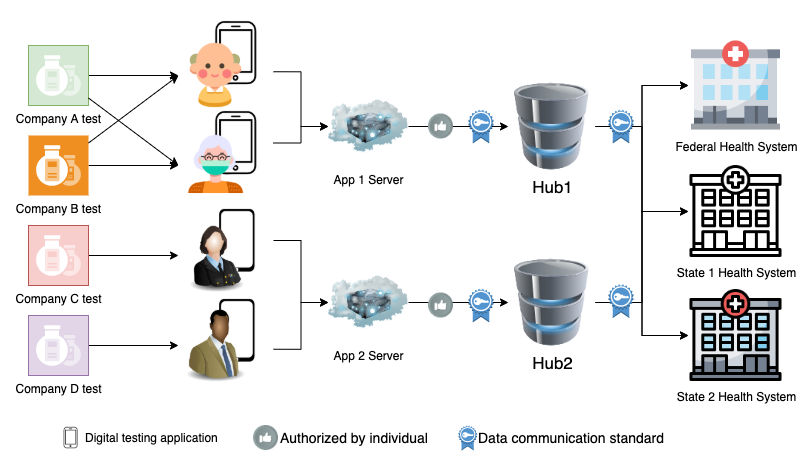
Definitions:
- Digital testing application - An application, such as a smartphone app or website, that accompanies a self-test that provides guidance on conducting a test properly and a mechanism to report results.
- Data communications standard - A uniform way of encoding test results and associated metadata. Healthcare industry standard communications include HL7v2 (Health Language 7 version 2) and FHIR (Fast Healthcare Interoperability Resources).
- Hub - A destination where results can be sent and subsequently re-transmitted to downstream state and federal public health systems. Also known as an 'exchange' or 'clearinghouse'.
Digital Testing Applications
Digital testing applications that currently support the MARS HL7v2 specification are listed below.
- iHealth Labs, Inc. - iHealth COVID-19 Rapid Antigen Test
- Detect, Inc. – Detect Covid-19 Test™
- Primary.Health - Multi-test support
- Workflow Services by ImageMover - Multi-test support
- CLX Health - Multi-test support
- Intrivo, Inc. - On/Go and On/Go One COVID-19 Tests
- CareEvolution, LLC - Multi-test support
- eMed, LLC - Multi-test support
- ACON Laboratories, Inc. - Flowflex COVID-19 Antigen Home Test
- QuidelOrtho Corp - QVue Digital Testing Platform + QuickVue At-Home COVID-19 Test
Hubs
Two hubs currently support the MARS HL7v2 specification:
- APHL Informatics Messaging Services (AIMS) - This hub, developed by the Association of Public Health Laboratories (APHL), has long been used to route laboratory test results to government health systems. More recently, AIMS has added support for COVID self-administered test reporting, including the MARS HL7v2 specification. AIMS currently connects to all US states and territories.
- ReportStream - This hub, created by the Centers for Disease Control and Prevention (CDC), is a free, open-source reporting solution for COVID test results. ReportStream securely stores and protects test results and patient data by two-factor authentication, database encryption, and HTTPS. Currently, ReportStream is connected to most federal, state, and local public health authorities, with a goal to integrate to all 64 public health authorities. In addition to handling reporting for laboratory and over-the-counter tests—self-administered or proctored—ReportStream has added support for the MARS HL7v2 specification.
Please visit this page for more details about the data hubs.
Data Communications Standards
HL7v2 and FHIR are two standards that can be used to encode test result messages. A comparison of these two communications standards, prepared in partnership with ONC, can be found here. Two implementation guides are available, one based on HL7v2 and another based on FHIR. The HL7v2 implementation guide is designed to be accepted by all states in the US. A FHIR implementation guide, while built and available, is not yet compatible with US public health systems.
Mobile Application Guidance
Mobile applications should encourage users to submit data for public health reporting while ensuring (1) transparency in how data is managed, (2) user control over data, and (3) ease of use in taking tests and reporting results. The minimum data required to permit reporting are test result, test date, age, and zip code. Users may voluntarily submit additional information such as demographic information.
See Mobile Application Guidance for more details.
MakeMyTestCount

MakeMyTestCount.org is website, developed by RADx®, that enables individuals to report at-home test results to state, local, and federal health agencies. MakeMyTestCount leverages the RADx MARS HL7v2 standard for all reporting.
