While pacemakers have been instrumental in treating many patients with heart rhythm disorders, their bulky design and dependence on wires can limit their usefulness and poses a risk of heart damage or infection. Researchers at the University of Chicago have cut the cords, shrunk the size, and expanded the capabilities of current designs.
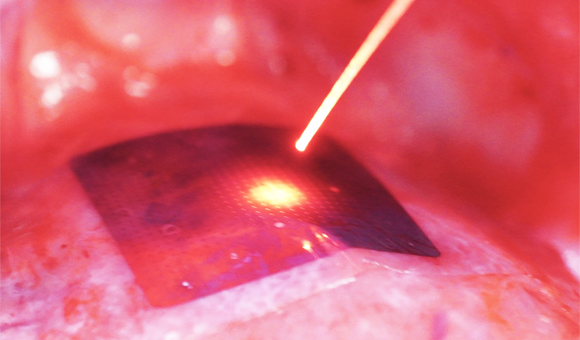
Their new technology is a lightweight, flexible silicon membrane that conforms to the surface of the heart, delivering electrical current when and where beams of light strike the device. In a recent study in Nature, the authors used their strategy to regulate heart activity in a variety of live animals. The results suggest the system could someday provide a high degree of control over irregular heart activity while placing less risk and burden on patients than traditional pacemakers.
The heart’s rhythmic motions are the result of electrical signals spreading through the tissue, causing the different chambers of the heart to contract in a particular order. Cardiovascular diseases or acute events, such as heart attacks, that injure heart tissue can diminish or disrupt this life-sustaining rhythm.
Current treatments usually involve the use of pacemakers that apply electrical stimulation to restore or correct heart activity. These devices typically deliver impulses through wires, or leads, surgically lodged into chambers of the heart.
“With traditional, lead-based pacemakers, stimulation is limited to where you implant the leads. But this is not ideal because sometimes the optimal location for stimulation changes over time,” said co-corresponding author Bozhi Tian, Ph.D., a professor of chemistry at the University of Chicago. “Basically, with our method, you have more freedom to adjust the stimulation location.”
This freedom stems from a process called the photovoltaic effect, wherein light rays cause a material to produce an electric current. The new pacemaker technology mirrors the structure of a solar cell — its two silicon layers primed to facilitate electrical flow — but instead of storing the electrical charge, the device distributes it onto the underlying heart muscle below.
In their study, Tian and colleagues first evaluated their pacemaker device in samples of cardiac cells and ex vivo animal hearts. By shining a laser on the membrane, they successfully triggered contractions in both models.
Next, they tested their method in live mice, rats, and pigs. After applying the device, the team stimulated heart tissue at various locations, sometimes simultaneously. This coordinated stimulation is critical for a heart failure treatment called cardiac resynchronization therapy (CRT). With CRT, physicians utilize pacemakers to restore and coordinate activity across the heart’s upper and lower chambers.
“Currently available pacemakers are capable of pacing at only one or two sites and often fall short in achieving synchronicity for about a third of patients receiving CRT,” said co-corresponding author Narutoshi Hibino, M.D., Ph.D., a professor of surgery at the University of Chicago Medical Center. “Pacing at multiple sites addresses this limitation by synchronizing movement across different areas of the heart.”
Throughout all experiments, the authors succeeded in stimulating hearts to beat steadily across a wide range of target rates using healthy animals. In one experiment they obtained similar results in an injured heart model. The outcome of these tests was promising but limited by the fact that they used a highly invasive approach to place the device on the heart and stimulate it with a light source.
The team devised a less intrusive procedure for their final test, conducted in a live pig. They inserted a collapsed version of their device through a small incision to position it on the heart. Then, the researchers slid an optic fiber through the same opening to shine light on the device and regulate the animal’s heart rate.
While the study proved that the new approach holds water for short-term procedures such as CRT, the authors believe that their device could find use in long-term applications. For treatment of chronic conditions, a small, wireless LED implanted under the skin of a patient could remotely trigger stimulation, Tian said.
The authors intend to perform experiments in animals over longer stretches of time to strengthen the case for their technique. Additionally, they plan to integrate their technology with skin-attached sensors for measuring heart activity — a capability needed for the system to provide stimulation at the right time.
“The authors not only have developed a new way to perform cardiac electrical stimulation but have also carefully considered how this method could translate to the clinic. It will be interesting to see how this technology evolves,” said Jessica Falcone, Ph.D., director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Medical Devices Program.
This research was partially supported by a grant from NIBIB (R56EB034289).
This science highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process—each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
Study reference: Pengju Li et al. Monolithic silicon for high spatiotemporal translational photostimulation. Nature. DOI: 10.1038/s41586-024-07016-9
