NIBIB funding drives progress in the diagnosis, treatment, and monitoring of middle ear infections
It’s a perennial pediatric problem: more than 80 percent of children will have at least one ear infection by their third birthday.
Beyond the pain and irritability that an ear infection can bring — for both children and their parents alike — untreated or recurring infections can result in hearing loss, which may affect the development of speech and language skills. With nearly 20 million estimated cases of acute middle ear infections each year in the United States, the annual cost associated with this condition is estimated to exceed $4 billion.
“It’s a universally understood misery, this issue of chronic ear infections in kids,” said NIBIB-funded researcher Stephen Boppart, M.D., Ph.D., the Grainger Distinguished Chair in Engineering at the University of Illinois at Urbana-Champaign. “When we talk with parents, nearly all of them identify with this problem, because it’s so prevalent.”
Boppart, who is also the executive associate dean and chief diversity officer at the Carle Illinois College of Medicine and a recipient of several grants from NIBIB, has spent more than a decade developing new ways to diagnose, treat, and monitor middle ear infections. His focus? Finding ways to detect and eradicate biofilms.

“This project highlights the transformative power of early-stage funding for biomedical imaging technologies,” said Behrouz Shabestari, Ph.D., director of the NIBIB National Technology Centers. “By supporting promising research in its infancy, NIBIB can help to facilitate the realization of novel ideas into practice-changing and translational advancements.”
A biofilm is a community of microorganisms — typically including bacteria — that has adhered to a moist surface and has taken up residence there. Organisms within the biofilm secrete compounds (such as polysaccharides, proteins, and nucleic acids) into their environment, which form a protective matrix and allow the biofilm to grow. Biofilms can live on things like catheters, contact lenses, implants, or other objects that have contact with our bodies. But biofilms also naturally occur within us — they line our intestinal tract, and they form plaque on our teeth. They can also grow behind our eardrums.
“Biofilms can become established in our ears after recurrent infections,” Boppart explained. “The scaffold that encompasses the biofilm can protect bacteria from antibiotics, and over time, the bacteria can develop resistance to these drugs. This is of great concern for treating future infections.”
Beyond antibiotics, surgical intervention — where drainage tubes are placed in the eardrum — is a common treatment for chronic ear infections. Research suggests that currently, surgery may be the optimal treatment for biofilm-mediated infections. However, clinicians don’t yet have the tools to see if a biofilm has developed in the middle ear, as this cavity lies beyond the eardrum. One of Boppart’s early innovations was to adapt an imaging technique to allow clinicians to visualize this largely inaccessible area.
Developing a diagnostic tool for biofilm detection
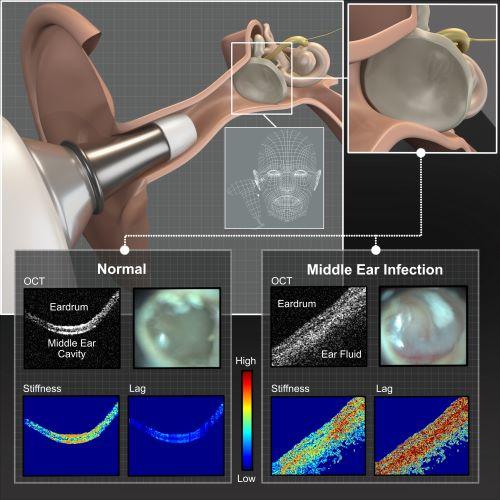
“The current tool used to diagnose an ear infection is a standard otoscope, which is essentially just a magnifier with a light source,” Boppart explained. “Because the clinician can’t see much beyond the eardrum, their diagnosis is based on subjective interpretation of whether or not fluid is present there. This can lead to unnecessary antibiotic use, or can prolong the time to necessary surgery,” he said. Importantly, otoscopes also can’t detect if a biofilm is present.
This is where Boppart sees optical coherence tomography, or OCT, coming into play. OCT is a non-invasive imaging technique that is analogous to ultrasound imaging, but instead of using sound waves and their reflections to visualize tissues inside the body, researchers use near-infrared light. With this technique, a clinician is able to obtain images beyond the eardrum — and see if fluid has built up in the middle ear cavity, or if a biofilm has developed.
Back in 2012, Boppart and his colleagues published a study in PNAS showing that OCT could be used to determine if fluid and/or biofilms were present in the middle ear cavity among patients with chronic ear infections. While the initial system was somewhat portable, it still took up a considerable amount of space. Now, he has refined and adapted this technology, and the entire system can fit inside a briefcase.
In this system, recently published in Biosensors, a small handheld probe is used to both deliver the light and to collect the associated reflections, which are used to generate cross-sectional images of the eardrum and the middle ear cavity. This allows the clinician to visualize if there is fluid in the middle ear, or if a biofilm is present behind the eardrum. Interpreting these images to make an accurate diagnosis would traditionally take experience and training, but Boppart and his colleagues have taken the next step: they have incorporated a real-time machine learning platform into their system.
“As long as the user can point the probe to the right location, the machine learning algorithm can detect if fluid or a biofilm is present in the middle ear,” explained Boppart. He noted that this system has the potential to improve diagnostic accuracy for ear infections, but beyond that, this early work implies that an accurate diagnosis can be made by users with varying levels of experience.
“Right now, clinicians are limited because they don’t have the right tools to see the infection beyond the eardrum,” Boppart explained. “And that’s what we’re giving them, really — a tool to see more about the disease, and then from that, to make better decisions in management and treatment.”
A new type of treatment for biofilm-mediated infections
OCT can allow a clinician to ascertain whether or not a biofilm is present in the middle ear. If so, they may opt for surgery instead of more antibiotics for treatment. While this surgical intervention is generally safe, the procedure requires general anesthesia and is not without risk.
But what if there was a way to destroy the biofilm — and eradicate the ear infection — that didn’t require surgery? Boppart and his team are investigating a treatment that sounds a little like something you’d hear discussed on an episode of Star Trek: a microplasma jet array.
In this approach, tiny electrodes are placed in a miniaturized, 3D-printed array that is filled with an inert gas. When a current is applied to the electrodes, the voltage ionizes the gas to produce free radicals, which are known to be bactericidal. These free radicals can diffuse across the eardrum into the middle ear cavity, where they can kill the bacteria and destroy the biofilm.
These cold microplasmas are under investigation in medicine for a variety of applications, including dental treatments and wound care, explained Boppart. “This new application has the potential to revolutionize the treatment of ear infections and reduce surgery in the pediatric population,” he said.

Boppart recently published a proof-of-concept study evaluating this microplasma jet array in the Nature journal, npj Biofilms and Microbiomes. Here, they tested whether microplasma could affect Pseudomonas aeruginosa, a common bacterial strain associated with middle ear infections, using an animal model and an artificial eardrum-mimicking membrane. In these experiments, they found that the microplasma treatment could inactivate P. aeruginosa.
“We built a prototype of our microplasma jet array, which we can attach directly to an otoscope, replacing the disposable tip,” explained Boppart. “But because the microplasma treatment could take up to 20 minutes, an alternative delivery platform could be an earbud — which kids are already used to using — and we could augment the microplasma treatment with music, or audio from a video. That’s a potential future of this technology.”
Important next steps will be to evaluate the microplasma jet array in additional animal models, and to find ways to monitor whether or not the treatment has worked in such models, and eventually, in humans.
A method to monitor response to treatment
“Monitoring response to treatment among children with ear infections has a variety of different challenges,” said Boppart. While pediatric patients may receive their initial assessment at their primary care provider, if their condition worsens, they make seek emergency care, he explained. On the other hand, if a child receives antibiotics and their symptoms dissipate, they often will not return for any sort of follow-up examination. In this way, collecting longitudinal information about response to treatment is notably difficult, he said.
As a first step down the path to noninvasive, longitudinal monitoring of response to treatment among children with ear infections, Boppart and colleagues evaluated their OCT system in a chinchilla model. In this study, recently published in the Nature journal Scientific Reports, the researchers analyzed the different characteristics of ear infections among the animals and evaluated how the chinchillas responded to antibiotics, using both OCT and standard otoscopy. By following the animals for three weeks, and imaging the middle ear cavity nearly every day, the researchers could compare the two diagnostic techniques and follow the infections in real time.
“Not only did this study reinforce the finding that OCT far exceeds standard otoscopy in evaluating the characteristics of a middle ear infection, it also sets the stage for the important longitudinal studies in human patients that will follow,” Boppart said.
From bench to bedside — to changing the standard of care
The term ‘bench to bedside’ is often used to describe translational research — that is, developing a technology in the lab that can then be used to help a patient. But being able to adapt that technology so that it can benefit the entire population — and ultimately transform the standard of care — is an equally challenging feat, Boppart said.
This, usually, is where biomedical industry steps in. Industry can help to take the technology from the limited patient studies to the larger clinical trials; to benchmark the method against the standard of care; and to facilitate the adoption and acceptance of the technique among clinicians. The more developed the technology is, the more appealing it will be for a company to take on this investment.

“With NIBIB funding, we were able to do those limited clinical studies, and we could build prototype OCT instruments that could be used in the clinic,” Boppart said. “This funding helped us to refine and to de-risk our technology, which could ultimately facilitate its adoption into clinics around the country, where it could transform the diagnosis and monitoring of middle ear infections for all.”
Boppart has also founded a startup company, called PhotoniCare, which is using his OCT technology to develop a commercially available middle ear scope. The company plans to launch the scope this year.
“This project highlights the transformative power of early-stage funding for biomedical imaging technologies,” said Behrouz Shabestari, Ph.D., director of the NIBIB National Technology Centers. “By supporting promising research in its infancy, NIBIB can help to facilitate the realization of novel ideas into practice-changing and translational advancements.”
Studies described herein were funded by grants R01EB013723 and R01EB028615 from the National Institute of Biomedical Imaging and Bioengineering.
