By manipulating cancer evolution, researchers destroy resistant tumors in animal model
Genetic diversity is key to survival in an everchanging environment. Cancer cells — thanks to their abundant and eclectic mix of mutations — unfortunately excel at surviving.
Drug treatments can diminish tumors for a time but, in many cases, give rise to resistant cells. A different drug may eliminate the resistant population during follow-up treatment only to leave behind a new resistant line that continues to proliferate. With patients having finite time and therapeutic options, tumor evolution can outpace the standard clinical treatment.
But what if instead of chasing after an evolving target, you could trick it into meeting you halfway?
“Our idea is basically to use the selection that occurs during cancer therapy as a tool to change the evolutionary trajectory in a way that makes treatment more effective,” said Justin Pritchard, Ph.D., an associate professor of biomedical engineering at the Pennsylvania State University.
Pritchard led a team of researchers in developing a strategy to genetically modify cancer cells with a pair of genes that would allow them to manipulate tumor evolution. In a study published in Nature Biotechnology, the researchers successfully employed the approach to eradicate tumors in a mouse model of lung cancer.
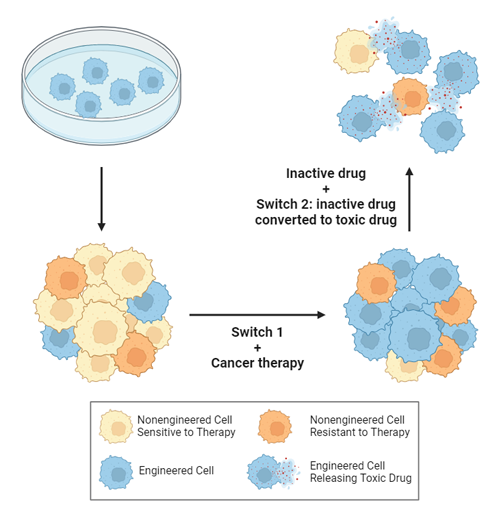
Past attempts to genetically engineer vulnerabilities into tumors fell short clinically, seemingly because of the difficulty in getting the genes into enough cells, Pritchard explained. His team wouldn’t deliver genes to many cells either. Instead, their plan was to expand the number of engineered cells by leveraging the selection that occurs during treatment with targeted therapies. Here’s how.
First, viruses are engineered to insert a pair of genes into cancer cells. Once the genes are inside, the researchers can turn them on like switches by delivering specific drugs to the tumor. The gene they activate first confers resistance to a particular drug that, when used to treat a tumor, destroys the native, unmodified cancer cells, leaving behind their genetically engineered counterparts.
Once the modified cancer cells become dominant in a tumor, the team switches off the first gene and turns their attention to the second one. They can activate this second gene by, for instance, introducing a drug precursor that the modified cells manufacture into a toxin, causing them to self-destruct and take out nearby, unmodified stragglers in the process.
The team used computational models to finetune their dual-switch system before testing it in the lab against a gauntlet of mutations associated with drug resistance in human cancer cell lines, Pritchard said.
After proving viable in cell culture, they moved their strategy into animals. The researchers transferred mixes of cancer cells to mice, with some cells being modified, while others were either naturally resistant or sensitive to a specific drug.
In one set of experiments, they implanted human non-small cell lung cancer (NSCLC) cells, which are notorious for becoming drug resistant. Mutations in a surface protein, called the epidermal growth factor receptor (EGFR), are a common source of this resistance. Physicians frequently use the targeted cancer drug osimertinib to control tumors with these mutations, but it often only helps temporarily, until new mutations emerge.
The researchers inserted dual-switch genes, one of which can provide osimertinib resistance, into a modest 10% of implanted NSCLC cells in a group of mice. For a comparison to clinical treatment, another group of mice were implanted with unmodified NSCLC cells.
All tumors shrank initially in response to osimertinib treatment, but then bounced back as resistant cells began to dominate. When the researchers flipped on the second switch in the modified tumor cells, this not only suppressed growth but completely wiped out the tumors in over 90% of the mice. However, the mice implanted with the unmodified cancer cells had continued tumor growth (as is typically seen in a clinical setting).
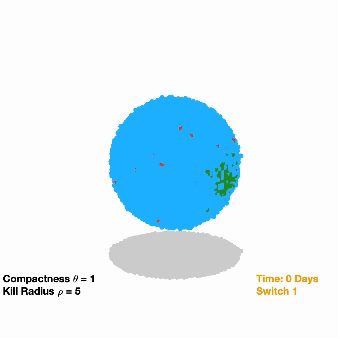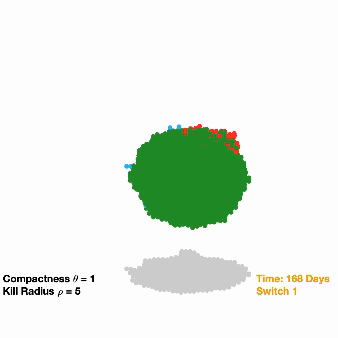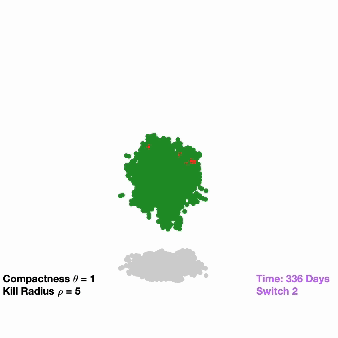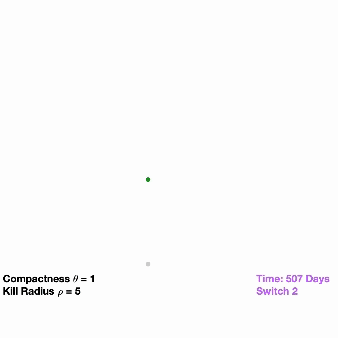
The researchers were encouraged by the results but are aware that several challenges remain before the technology reaches the clinic, Pritchard explained. For example, in the study they genetically modified cells outside of the body but, in the future, hope to get the dual-switch genes into patient tumors directly.
“The authors engaged in highly rigorous testing across in silico, in vitro, and in vivo experiments, and their exciting results have the potential to push dual-switch gene drives to further impact cancer research,” said David Rampulla, Ph.D., director of the Division of Discovery Science and Technology at the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
This research was supported in part by grants from NIBIB (R21EB026617) and the National Cancer Institute (NCI; U01CA265709).
For more coverage of this study, visit the NCI Cancer Currents blog.
This science highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process—each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
Study reference: Scott M. Leighow et al. Programming tumor evolution with selection gene drives to proactively combat drug resistance. Nature Biotechnology. DOI: 10.1038/s41587-024-02271-7
