Vascular and Orthopedic Complications of Sickle Cell Disease
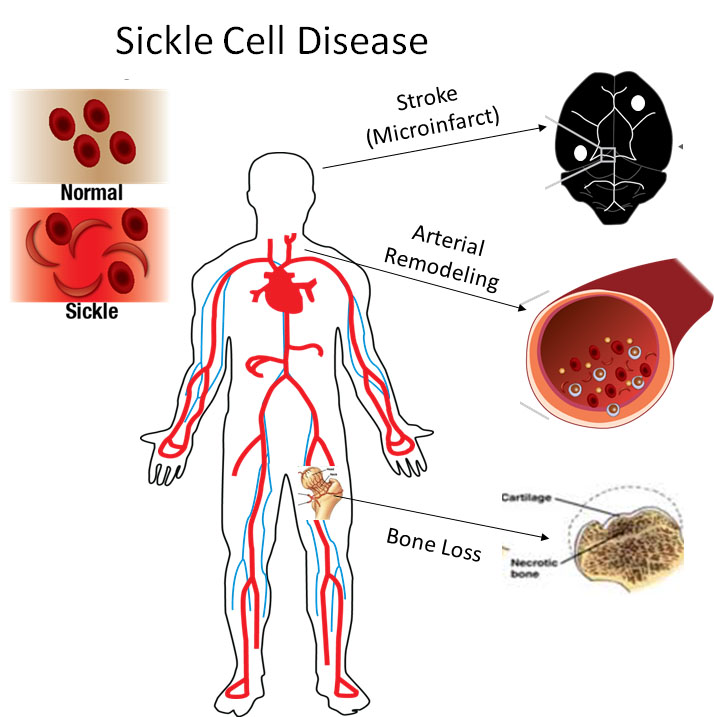 Children with sickle cell disease have an 11% chance of suffering a major stroke by the age of 16, but 33% are at risk of having a silent stroke that impairs mental function and cognitive abilities. We are investigating the cellular and inflammatory mechanisms accelerating these destructive tissue remodeling.
Children with sickle cell disease have an 11% chance of suffering a major stroke by the age of 16, but 33% are at risk of having a silent stroke that impairs mental function and cognitive abilities. We are investigating the cellular and inflammatory mechanisms accelerating these destructive tissue remodeling.
Computational Fluid Dynamics of Blood Flow in the Cerebrovasculature
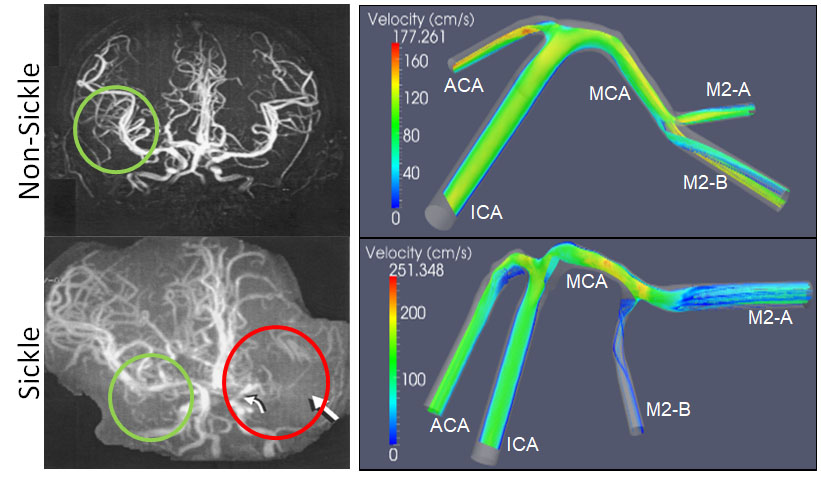 Hemodynamic parameters of the blood in sickle cell disease change its properties and can influence cell and tissue mechanic properties in the vasculature. Computational fluid dynamics models of disturbed blood flow in these individuals are currently being developed using human data as well as transgenic mouse models in our research laboratory and with our collaborators
Hemodynamic parameters of the blood in sickle cell disease change its properties and can influence cell and tissue mechanic properties in the vasculature. Computational fluid dynamics models of disturbed blood flow in these individuals are currently being developed using human data as well as transgenic mouse models in our research laboratory and with our collaborators
Sickle Cell Large Artery Biomechanics and Imaging
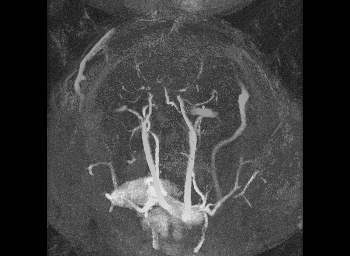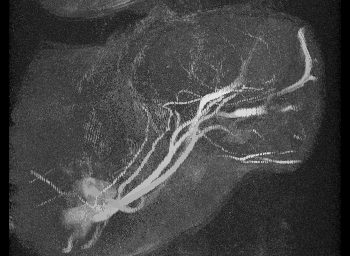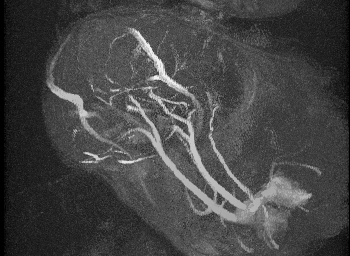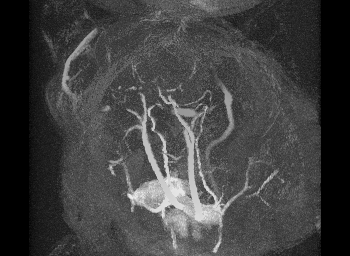 Mechanical testing of carotid arteries and aortas from mice with sickle cell disease allows us to understand the changes to the elastin and collagen structures as they are remodeled due to upregulation of cathepsins in response to sickle cell disease. Cerebral arteries are more difficult to visualize so we use a label free method of magnetic resonance angiography.
Mechanical testing of carotid arteries and aortas from mice with sickle cell disease allows us to understand the changes to the elastin and collagen structures as they are remodeled due to upregulation of cathepsins in response to sickle cell disease. Cerebral arteries are more difficult to visualize so we use a label free method of magnetic resonance angiography.
Aggressive Breast Cancer in Young Women in Ethiopia
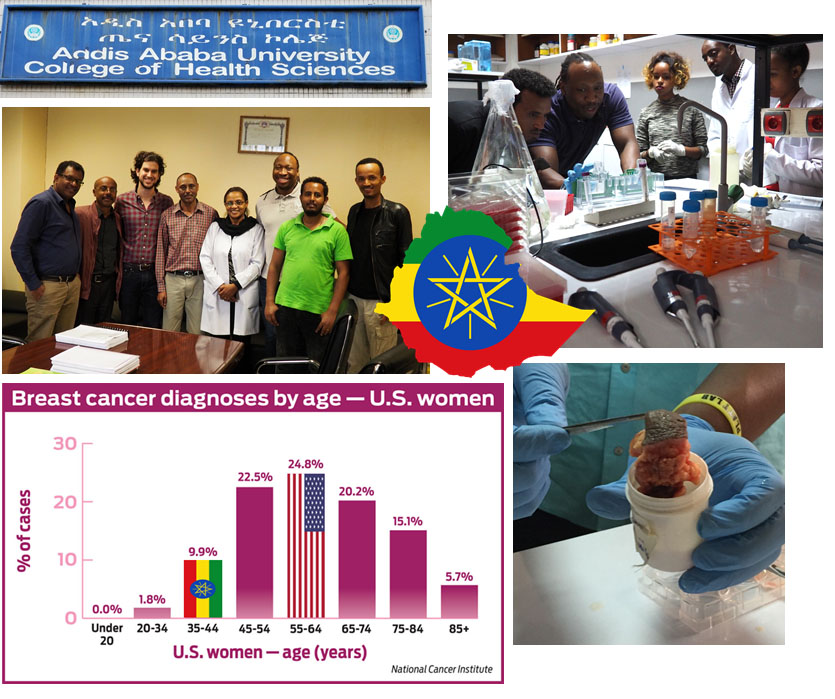 Women in Ethiopia develop extremely aggressive breast cancer, and this can occur at very young ages such as their 20s or 30s. Our hypothesis is that tumors in Ethiopian women are more proteolytically active leading to aggressive growth and invasion. Proteolytic profiling would determine why some women present with more advanced tumors at earlier ages, but low cost methods are needed for practical translation to this beautiful country.
Women in Ethiopia develop extremely aggressive breast cancer, and this can occur at very young ages such as their 20s or 30s. Our hypothesis is that tumors in Ethiopian women are more proteolytically active leading to aggressive growth and invasion. Proteolytic profiling would determine why some women present with more advanced tumors at earlier ages, but low cost methods are needed for practical translation to this beautiful country.
Cathepsin Proteolytic Networks
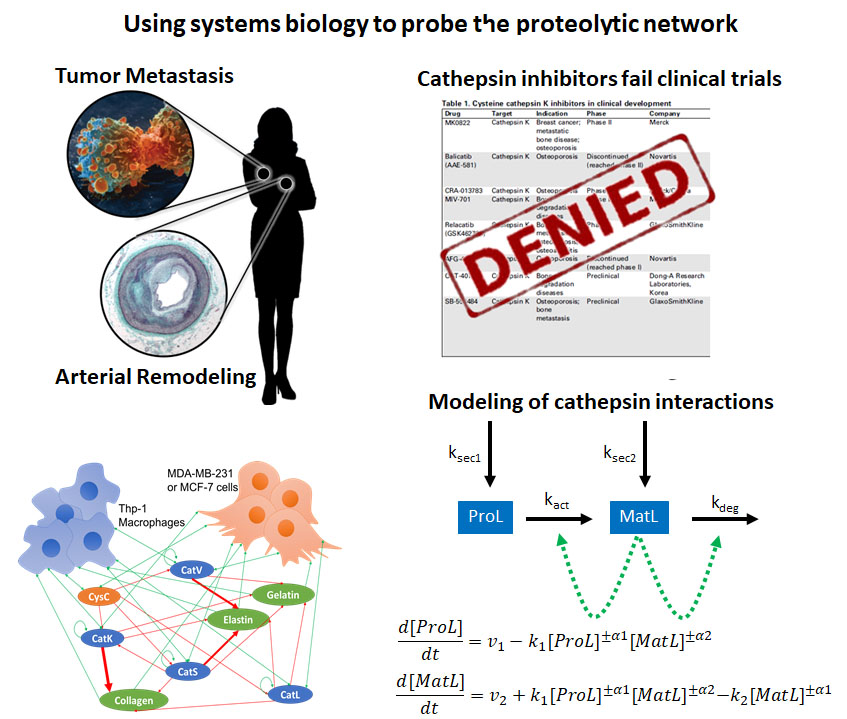
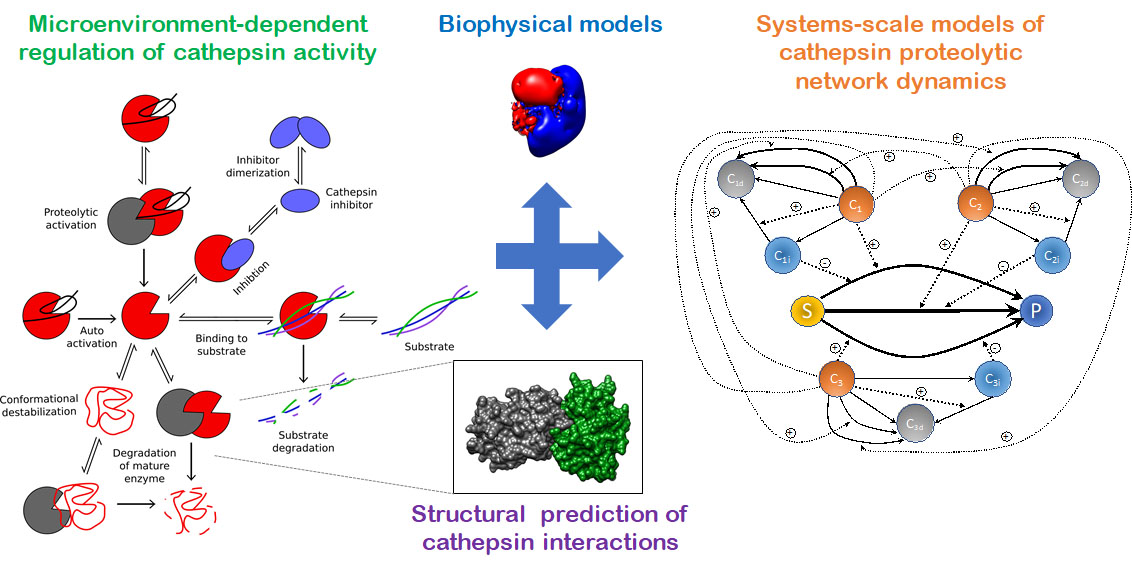 Cysteine cathepsins K, L, S, and V are part of a complicated network of proteases and inhibitors that work with and against each other to degrade extracellular matrix and are upregulated in a range of tissue destructive diseases. Our overall goal is to develop integrated, dynamic models of both extra- and intra-cellular cathepsin production and activity that can predict shifts to the proteolytic network caused by inhibitors or mutant proteases.
Cysteine cathepsins K, L, S, and V are part of a complicated network of proteases and inhibitors that work with and against each other to degrade extracellular matrix and are upregulated in a range of tissue destructive diseases. Our overall goal is to develop integrated, dynamic models of both extra- and intra-cellular cathepsin production and activity that can predict shifts to the proteolytic network caused by inhibitors or mutant proteases.
Personalized Proteolytic Potential
 Patient-to-patient variability in disease progression continues to complicate treatment decisions for cardiovascular diseases and metastatic cancers. By demonstrating a range of patient-to-patient variability in cathepsin activity from patients’ macrophages, we developed the hypothesis that these differences, which may help cancer spread and metastasize, could be detected via a blood test. Using systems biology techniques, our goals are to predict patient prognoses for tissue destructive diseases.
Patient-to-patient variability in disease progression continues to complicate treatment decisions for cardiovascular diseases and metastatic cancers. By demonstrating a range of patient-to-patient variability in cathepsin activity from patients’ macrophages, we developed the hypothesis that these differences, which may help cancer spread and metastasize, could be detected via a blood test. Using systems biology techniques, our goals are to predict patient prognoses for tissue destructive diseases.
