Collaborators
Project Brief
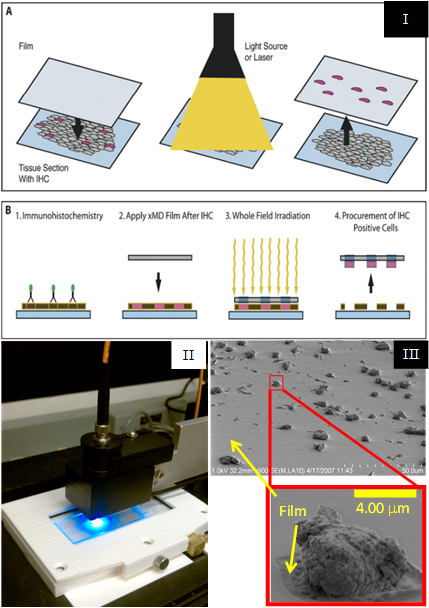
Laser Capture Microdissection (LCM) is a well-established technology used to isolate cells of interest from surrounding tissue cells on a microscope slide. As early co-inventors of LCM in the mid-90s, SPIS staff have continued to develop innovative tissue microdissection technologies, working with partners in NCI, NICHD, NIBIB, NIMH, NIDA, and industry. Although LCM is already commercially successful, the method requires a skilled operator to select the cells for capture, which leads to operator variability and limits overall throughput. In more recent years, SPIS and collaborators have developed Target Activated Microdissection (TAM) to address these limitations associated with some applications. TAM uses targeted molecular probes and light-absorbing tissue stains that generate localized heat under suitable illumination, bonding the desired cells to a thin thermoplastic placed in contact with the tissue. The initial TAM prototype used a scanned laser to illuminate the entire tissue section, in which the following performance enhancements were achieved:
- Increased dissection rate by orders of magnitude
- Increased dissection precision to the subcellular level
- Removed requirement for operator target selection, permitting process standardization
- Eliminated targeting difficulties due to poor image quality of histology sections
- Maintained spatial relationship of tissue morphology, allowing better image documentationStephen Hewitt, MD, PhD
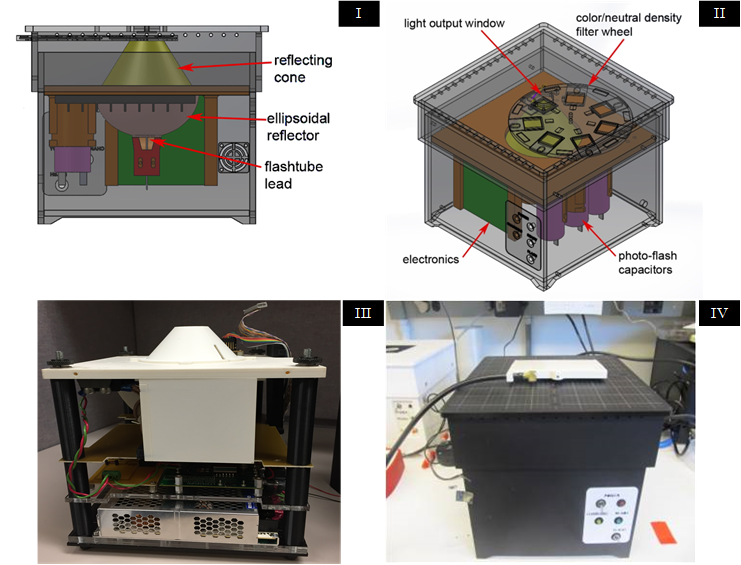
To further improve TAM performance, SPIS designed and produced a custom light source that delivers a brief, high-power, burst of light over the entire surface area of the tissue, reducing the time required to dissect tissue from a whole slide with subcellular precision to under a minute. During validation studies, SPIS introduced a variety of TAM instrumentation features, such as the ability to use multiple pulses in quick succession, the ability to alter the spectrum of light with filters, and the ability to save and reload settings for repeatable experiments. TAM developments have resulted in patents, licensing, and MTAs.
Proteomic analysis of nuclei dissected from fixed rat brain tissue using expression microdissection
Immunoguided Microdissection Techniques
Expression microdissection adapted to commercial laser dissection instruments
Expression Microdissection: A Novel Immuno-Based Dissection Technology for Cellular and Nuclear Procurement (unavailable)
Expression microdissection: operator-independent retrieval of cells for molecular profiling
Laser Capture Mircrodissection of Single Cells From Complex Tissues
An Instrument for Performing Laser Capture Microdissection of Single Cells (unavailable)
Tech Transfer
Patent licensing: XMD Diagnostics, LLC
MTAs with industry partners for internal use and commercialization
CRADA negotiations with industry partner for pre-licensing evaluation
Technology Dissemination: Expression Microdissection highlighted as a ‘New Methods’ article in the ‘NIH Catalyst’, a publication about NIH intramural research
International patent application: Device for Selectively Removing Cells from a Biological Sample
Patent: Target activated microtransfer
Patent: Method patent: Target activated microtransfer
Patent: Device patent: Target activated microtransfer
Patent: Method of laser capture microdissection from a sample utilizing short pulse length
Patent: Non-contact Laser Capture Microdissection
Patent: Convex geometry adhesive film system for laser capture microdissection
Patent: Mechanical Handling Systems for Laser Capture Microdissection
Patent: Precision laser capture microdissection utilizing short pulse length
Patent: Method utilizing convex geometry for laser capture microdissection
