Collaborators
Project Brief
Retinal degenerative diseases currently have limited treatment options partly due to a lack of effective in vitro model systems to investigate mechanisms of disease pathogenesis and evaluate new therapies. To face this challenge, research has been conducted to culture three-dimensional (3D) retinal organoids from mammalian pluripotent stem cells (PSC). In static culture, PSC-derived neural retina (NR) mimics in vivo mouse retina with proper polarity and limited stratification of major cell types. However, these NRs are still limited by the immaturity of their photoreceptors, which do not develop the outer segment structures necessary for capturing light photons. In in vivo retina, outer segments are embedded in the retinal pigment epithelium (RPE) for structural support and nutrient transport; thus, co-culturing NR with RPE tissue may further differentiate photoreceptors. The proposed retinal organoids may generate photoreceptor cells suitable for cell replacement therapies that could potentially restore vision. Furthermore, taking advantage of the indefinite proliferation and capacity to differentiate into various tissue types, this co-culture technology for PSC-derived organoids could enhance the methodology investigators use to study disease pathogenesis in other organ systems.
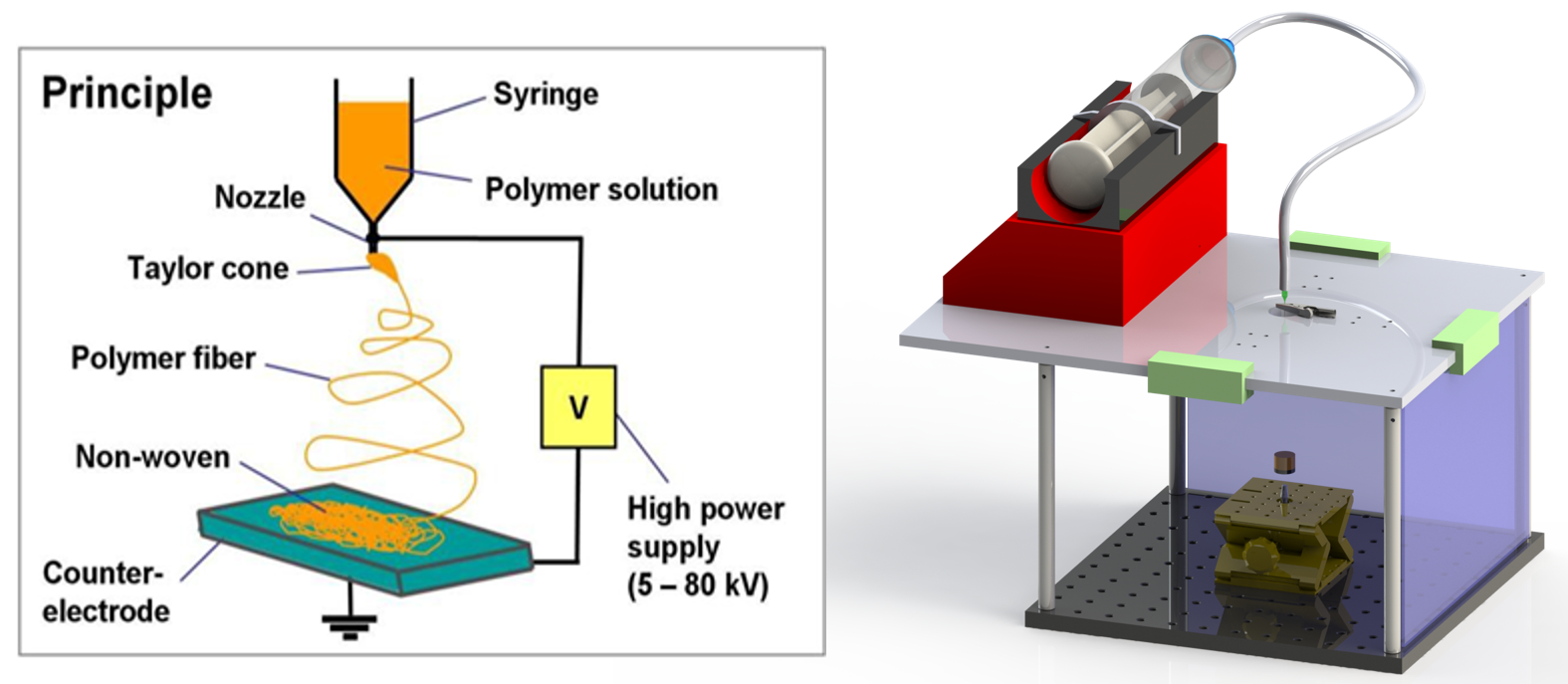
Bruch’s membrane is a 2-4 µm thick extracellular matrix located between the RPE and choroid layers. The membrane provides structural support and facilitates nutrient transfer to the RPE. The membrane has major clinical significance because of its role in retinal degenerative diseases, such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP). In collaboration with NEI and NIBIB, SPIS engineers are developing an electrospinning system to generate a nanofibrous scaffold material from poly (ε-caprolactone) (PCL). The PCL nanofibrous scaffold will be used to culture a PSC-derived RPE monolayer.